A research team led by Edwin CHEUNG, Professor in the Faculty of Health Sciences (FHS), University of Macau (UM), has reported the discovery of a novel protein factor responsible for promoting prostate cancer growth. The result of a study conducted by the team suggests that this protein factor is a promising therapeutic target in prostate cancer as inhibiting its expression will significantly affect the occurrence and progression of the disease. The research result was recently published in the internationally renowned journal EMBO Reports.
Prostate cancer is the most common type of cancer found in men and is also the second-leading cause of cancer death among males. The androgen receptor (AR), a hormone-regulated master transcriptional regulator, is responsible for the initiation and progression of prostate cancer. AR transcriptional activity is meticulously controlled by the physical and functional interactions with transcriptional co-factors. Changes in the expression or activity of any of these co-factors in prostate cancer will significantly affect the occurrence and progression of the disease. Thus, identifying and understanding the role of AR co-factors in prostate cancer is key to developing novel diagnostics and therapeutic strategies to combat this ailment.
In this study, the research team used a proteomics technique known as Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) to systematically identify all proteins interacting with AR in prostate cancer cells. They found that Tripartite motif-containing-motif TRIM33 (TRIM33) is a critical component of the AR transcriptional regulatory network, acting as a positive regulator of AR-mediated transcription. They also demonstrated through a series of detailed mechanistic experiments that AR recruits TRIM33 to specific genomic locations and stabilises AR protein levels by protecting AR from Skp2-mediated ubiquitination and proteasomal degradation. These events ultimately lead to enhanced AR binding to chromatin and the transcriptional activation of downstream target genes that control DNA metabolism, cell cycle, mitosis, and prostate tumor-related signaling pathways. The results indicate that TRIM33 plays a tumour-promoting role in prostate cancer.
Prof. Cheung’s team analysed the clinical data from prostate cancer patients. First, they found a significant increase in TRIM33 mRNA and protein levels in human prostate tumour tissue compared to normal prostate tissues. The researchers then defined an AR target gene directly co-regulated by TRIM33, and found that this gene set was highly expressed in prostate tumours and that patients with high expression of this gene signature are at a higher risk of disease recurrence. Therefore, the inhibition of TRIM33 expression makes prostate cancer cells more sensitive to AR antagonists. Together, these results suggest that TRIM33 is a promising therapeutic target in prostate cancer.
Prof. Cheung is the corresponding author of the study. His PhD students, namely Mi CHEN and Shreyas LINGADAHALLI, as well as former research assistant Nitin NARWADE, are co-first authors of the study. FHS Associate Professor Terence, Chuen Wai POON and his team also made important contributions to this study. The project was funded by the Science and Technology Development Fund, Macao SAR (File no: 102/2015/A3, 0011/2019/AKP) and UM (File No: MYRG 2018-00033-FHS and MYRG2020-00100-FHS). The full version of the paper can be viewed at: https://www.embopress.org/doi/full/10.15252/embr.202153468.
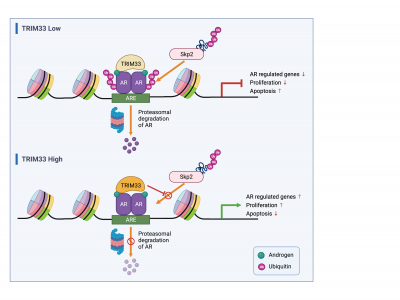
TRIM33 promotes prostate cancer progression by protecting androgen receptor from Skp2-mediated degradation.
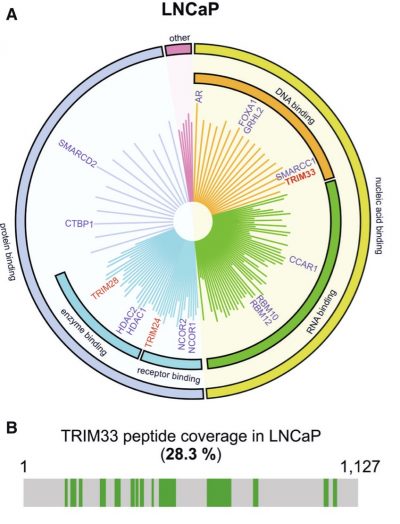
TRIM33 is a novel protein interactor of AR – A. A graph showing AR-associated proteins identified by RIME analysis in prostate cancer cells. B. Mass spectrometry peptide coverag

Edwin CHEUNG

Mi CHEN

Shreyas LINGADAHALLI

Nitin NARWADE

