The University of Macau (UM) has made new progress in breast cancer study. A team in the university’s Faculty of Health Sciences (FHS) has discovered that NOTCH1 activation can promote the growth of triple-negative breast cancer (TNBC). Titled ‘NOTCH1 Activation Compensates BRCA1 Deficiency and Promotes Triple-Negative Breast Cancer Formation’, the study has been published in the internationally renowned journal Nature Communications.
Led by Chuxia DENG, dean of the FHS, the research team has found that NOTCH1 activation can rescue BRCA1 deficiency and promote TNBC formation. Drugs targeting NOTCH1-regulated pathways can effectively inhibit the growth of TNBC. This mechanism may explain the discrepancies regarding the role of NOTCH1 as a tumour suppressor or an oncogene. For example, under some other conditions, such as when a tumour has intact DNA damage repair machinery or cell cycle checkpoints, the activation of ATR-CHK1 signaling by NOTCH1 should inhibit cell cycle progression and elicit its tumour suppressor functions to block the tumour development. More importantly, this finding has provided potent druggable targets for TNBC.
Breast cancer gene 1 (BRCA1), the first identified breast cancer susceptibility gene, is responsible for hereditary breast cancers. Inherited mutations in the BRCA1 gene predispose carriers to early-onset tumourigenesis and an up to 87 per cent cumulative lifetime risk of developing breast cancer and/or ovarian cancer. Moreover, approximately 48 per cent to 66 per cent of BRCA1 mutation carriers eventually develop triple-negative breast cancer (TNBC). TNBC is a cancer that tests negative for estrogen receptors (ER), progesterone receptors (PR), and HER2 protein. It is considered to be aggressive, unresponsive to hormonal therapy, and has a poor prognosis.
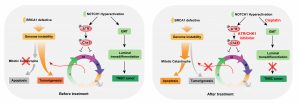
BRCA1 is crucial for multiple biological processes, including DNA damage repair, cell cycle checkpoints, ubiquitination, and transcriptional regulation. Studies have demonstrated that the absence of BRCA1 leads to genome instability, which then initially triggers the lethal block by inducing mitotic catastrophe and apoptosis. By using the Sleeping Beauty transposon system in BRCA1-deficient mice, the research team identified 169 putative cancer drivers, among which Notch1 is a top candidate for accelerating TNBC. They have found that the activation of NOTCH1 has at least two important functions in BRCA1 associated tumorigenesis: 1) it accelerates TNBC formation by promoting epithelial-mesenchymal transition (EMT); and 2) through a non-canonical target ATR-CHK1 axis, it restores S/G2 and G2/M cell cycle checkpoints, which are impaired due to Brca1 deficiency, to suppress the mitotic catastrophe, and thus benefits BRCA1 associated tumorigenesis. To cure TNBC, the researchers tested the combined treatment of targeting the NOTCH1 non-canonical target ATR-CHK1 cascade and the EMT, which are downstream of NOTCH1 signaling based on the mechanism they uncovered. The results demonstrated that the combination of cisplatin and ATR-CHK1 inhibitors effectively inhibits TNBC through rebooting the mitotic catastrophe, providing a potential therapeutic strategy for this deadly disease.
The study was led by FHS Dean Chuxia DENG, and Research Assistant Professor Kai MIAO is the first author. Associate Professor Xiaoling XU and Assistant Professor Koon Ho WONG also made important contributions to this study. The study was funded by the Science and Technology Development Fund, Macao SAR (File no. 094/2015/A3, 0011/2019/AKP, 0034/2019/AGJ, 0048/2019/A1, 029/2017/A1, 0101/2018/A3, and 111/2017/A) and UM’s research fund (File no. MYRG2016-00139-FHS and MYRG2018-00186-FHS). The full version of the related paper can be viewed at: https://doi.org/10.1038/s41467-020-16936-9
The molecular mechanism of NOTCH1 promotes BRCA1-related triple negative breast cancer and its targeted therapy
Chuxia DENG (right) and Kai MIAO