A research team lead by Prof. Kathy Qian LUO in the University of Macau (UM) Faculty of Health Sciences (FHS) has made a new progress in the development of animal model for tumour immunotherapy. The research team has generated a zebrafish model for the real-time observation of natural killer (NK) cell-mediated killing of tumour cells. This model can serve as a powerful in vivo tool for the evaluation of therapeutic effects of NK-cell-based immunotherapies. The research results have been published in the internationally renowned journal Biosensors and Bioelectronics.
In recent years, NK-cell based-immunotherapies have attracted the attention in the field. NK cells can kill tumour cells directly without antigen recognition. Compared to T cell therapy, NK cell-based therapy does not easily attack healthy cells of the host and has fewer potential side effects. Thus, various types of therapeutic NK cells are currently being developed, including cord blood NK cells, stem cell-derived NK cells, cytokine-induced memory-like NK cells, and CAR NK cells. The therapeutic effects of these NK cells need to be evaluated in appropriate animal models which can greatly facilitate the development of NK cell-based therapy.
In the previous research, using genetic engineering, research team developed a fluorescence resonance energy transfer (FERT)-based protein fluorescent probe, sensor C3, for real-time tracking of apoptosis. Sensor C3 emits green fluorescence in live cells and emits blue fluorescence in apoptotic cells. The researchers used sensor C3 to label tumour cells and a red fluorescent protein (tdTomato) to label NK-92MI cells and established an in vitro 3D model to study the killing of tumour cells by NK cells. The results showed that, in tumour cells and NK cells coculture group, 6 hours later, there were already lots of apoptotic tumour cells, and 36 hours later, almost all the tumour cells were killed by NK cells. In the tumour cell alone group, there were few apoptotic tumour cells.
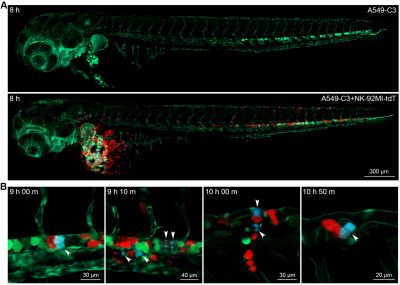
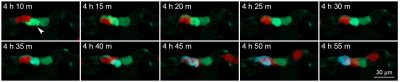
The research team then established an animal model that allowed the real-time observation of NK cell-mediated killing of tumour cells. Using a microinjection system, green tumour cells and red NK cells were injected into blood circulation of zebrafish larvae. Six hours after the injection, some tumour cells were observed to migrate to the trunk and tail region of zebrafish, and there were lots of apoptotic tumour cells. In contrast, very few apoptotic tumour cells were observed when tumour cells were injected alone. The statistical results showed that the apoptotic peak was 12 hours after injection and that NK cells could kill most of the tumour cells within 36 hours. Whole zebrafish imaging clearly showed the distribution of tumour cells and the killing of tumour cells by NK cells. High-resolution imaging revealed the interaction between NK cells and tumour cells at single-cell level. For example, NK cells could tightly bind to tumour cells or even change their morphology for a better killing. In addition, real-time imaging captured the dynamic killing process. The research team found that NK cells could recognize single tumour cell and induce its apoptosis within 40 minutes. All these results showed that this sensor zebrafish model can serve as a powerful in vivo tool to facilitate the development of NK cell-based immunotherapies.
Prof. Kathy Qian LUO is the corresponding author of the study, her PhD student Hongmei YANG and postdoctoral researcher Hao JIA are the co-first authors. FHS Associate Professor Qi ZHAO contributed to the research work. The Animal Research Core and the Biological Imaging and Stem Cell Core in FHS provided important support to this study. The project was supported by UM (file number: MYRG 2020-00121-FHS). The full version of the research article can be viewed at https://www.sciencedirect.com/science/article/pii/S095656632200656X.