A research team led by Qi ZHAO, Associate Professor at the University of Macau (UM) Faculty of Health Sciences (FHS) and the team led by Qingming SHEN, Professor at Nanjing University of Posts and Telecommunications, have made significant process in the development of tumour microenvironment (TME) activated NIR-II phototheranostic nanoplatform. They have successfully fabricated a TME-activated nanoprobe through a simple coordination-driven self-assembly strategy, which can achieve sensitive and specific tumour diagnosis by high tumour-to-normal tissue signal ratio and simultaneously synergistic anti-tumour therapeutic effect in the second near-infrared window. The research has been published in Small.
The phototheranostics in the second near-infrared window (NIR-II, 1000-1700 nm), have shown great prospects in tumour theranostics due to the depressed photon absorption and scattering by biological samples, deeper tissue penetration and higher maximum permissible exposure to lasers. However, most current NIR-II probes are in an “always on” mode, which emit invariable fluorescence signals in both lesion and normal tissues owing to their nonspecific effects. Such “always on” signal mode possibly leads to the low tumour-to-normal tissue signal ratio, limited sensitivity and specificity, and even “false positive” results. The non-specific diagnosis and treatments may cause irreversible damage to normal tissues during the off-target theranostic period.
Some activatable NIR-II phototheranostic probes have been fabricated to precisely distinguish lesions from normal tissues in response to TME. Despite excellent diagnostic specificity, current activatable NIR-II probes are mainly limited by complex chemical synthesis steps. In addition, to deliver the fluorophore, it may be necessary to introduce excess components, which might cause some excipient side effects. Therefore, it is highly desirable to develop a facile method to fabricate TME activatable NIR-II phototheranostic systems for improving the diagnosis specificity and therapeutic efficacy toward tumours.
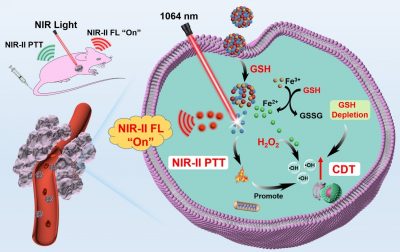
In view of this, the research team developed a TME-activated phototheranostic nanoplatform (AFD NPs) based on the principle of Förster resonance energy transfer (FRET). The AFD NPs are fabricated through self-assembly of Ag2S QDs (NIR-II fluorescence probe) and ultra-small semiconductor polymer dots (DBZ Pdots, NIR-II fluorescence quencher) utilizing Fe(III) as coordination nodes. In normal tissues, the AFD NPs maintain in “off” state, due to the FRET between Ag2S QDs and DBZ Pdots. However, the NIR-II fluorescence signal of AFD NPs can be rapidly “turned on” by the overexpressed glutathione (GSH) in tumour tissues, resulting in significantly enhanced tumour-to-normal tissue (T/NT) signal ratio for tumour-specific diagnosis. Moreover, the released Pdots and reduced Fe(II) ions provide NIR-II photothermal therapy (PTT) and chemodynamic therapy (CDT), respectively. The GSH depletion and NIR-II PTT effect further aggravate CDT mediated oxidative damage toward tumours, achieving the synergistic anti-tumour therapeutic effect. The work provides a promising strategy for the development of TME activated NIR-II phototheranostic nanoprobes.
Prof. Qi ZHAO and Prof. Qingming SHEN are the corresponding authors of the study, and UM postdoctoral fellow Yeneng DAI is the first author. The project was supported by the Science and Technology Development Fund of Macau (File nos. FDCT/0043/2021/A1 and FDCT/0002/2021/AKP), the National Key R&D Program of China (2019YFA0904400) and UM (File no. MYRG2022-00143-FHS). The full version of the research article can be viewed at https://onlinelibrary.wiley.com/doi/full/10.1002/smll.202206053.