A collaborative study led by Leo, Tsz On LEE, associate professor in the University of Macau (UM) Faculty of Health Sciences (FHS), and Ling PENG, professor in the Aix-Marseille University (AMU), has developed a novel small activating RNA (saRNA) to modulate G protein-coupled receptor (GPCR) expression, which provides a new strategy for cancer therapy and is expected to effectively control the growth and spread of different cancer cells. The study has been featured on the cover of Advanced Science.
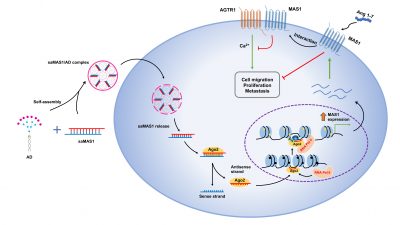
The research team used saRNA as a targeted drug for a GPCR and investigated the potential of this new approach in cancer treatment. The target GPCR, Mas receptor (MAS1), can modulate the renin-angiotensin system (RAS). RAS plays a fundamental role in multiple physiological systems and is involved in cancer progression and metastasis. The dysregulation of RAS affects tissue remodelling, inflammation, angiogenesis, apoptosis, and other processes and will exacerbate the malignancy of a tumour. For this reason, the team proposed targeting RAS as a novel approach to control the tumour microenvironment as well as tumour growth and spread. In the study, the researchers found that amphiphilic dendrimer (AD) could effectively deliver MAS1-saRNAs (saMAS1) to tumours, which led to a sustained enhancement of MAS1 expression in multiple cancer cell lines and models. The increase in MAS1 expression inhibited the signalling pathway of angiotensin II and its receptor (AGTR1) through ligand-dependent and non-dependent mechanisms, resulting in a significant suppression of tumourigenesis and related development.
GPCRs are the most common and important drug targets, and more than 30 per cent of drugs approved by the United States Food and Drug Administration (FDA) target GPCRs. However, these FDA-approved drugs only target around 27 per cent of non-olfactory GPCRs, meaning that a large number of GPCRs are currently undruggable. Furthermore, the specificity of conventional chemical agonists/antagonists of GPCRs is a major concern in drug development due to the various subtypes and splice variants as well as the high similarity of GPCRs within the same subfamily. Therefore, a new strategy is urgently needed to develop effective and specific drug candidates for the targeting of GPCRs.
Small nucleic acid molecules, such as small interfering RNAs (siRNAs) and saRNAs, can target any gene and modulate gene expression in a potent and sequence-specifically via Watson-Crick base-pairing. This approach offers unique advantages to address novel drug targets, including the undruggable GPCRs. In contrast to siRNA that inhibits gene expression, saRNA is a genetic approach to enhance gene expression. saRNA can recruit RNA-induced transcription initiation (RITA) complexes and use their antisense chains to pair with promoter regions of target genes, thereby stimulating proximal gene transcription initiation. Currently, the first saRNA drug (MTL-CEBPA) has been used in the evaluation of advanced liver cancer and has demonstrated clinical proof of targeting, and upregulating, the master myeloid transcription factor CEBPA (CCAAT enhancer binding protein alpha).
By proposing the use of saRNA as a targeted drug for GPCRs, this study provides not only a new avenue to address GPCRs that are currently undruggable, but also a new strategy for cancer therapy by targeting RAS. The study demonstrated that an saRNA-mediated increase of MAS1 expression is an effective approach to inhibit cancer growth in patient-derived tumour models and should have broad relevance in cancer treatment. Additionally, because of the pleiotropic role of RAS in multiple physiological processes, the saRNAs developed in this study may also be extended to treat other diseases, such as cardiovascular or COVID-19-associated diseases.
Prof. Lee is the co-corresponding author and the last author of this study, and his PhD student, Yunfang XIONG, is the first author. Other PhD students, namely Ran KE , Qingyu ZHANG, and Wanjun YAN, and undergraduate student Nga Ieng CHAN, also made important contributions to this study. This study is also the result of a collaboration between several FHS research groups, with members including Prof. Sup Shim JOONG, Prof. Xuanjun ZHANG, and Prof. Ruiyu XIE. The project was supported by the Science and Technology Development Fund, Macao SAR (File no: 0016/2020/A1) and UM (file number: MYRG2018-00161-FHS). The full version of the research study can be viewed at https://onlinelibrary.wiley.com/doi/10.1002/advs.202200562.