A research team led by Assistant Professor Dai Yunlu in the University of Macau (UM) Faculty of Health Sciences (FHS) has designed and synthesised a novel nonomedicine, which can evoke potent anti-tumour immunoresponse, inhibit tumour growth, and stimulate long-lasting immunological memory. The study is a new breakthrough in melanoma immunotherapy and proposes a promising anti-tumour strategy. The research findings have been published in the internationally renowned journal Nature Communications.
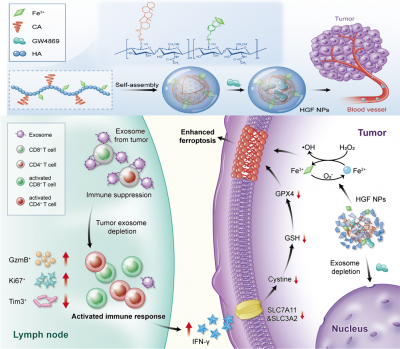
Tumour cells can evoke systemic immune perturbations for rapid expansion. Beyond upregulating the expression of programmed death-ligand 1 (PD-L1) on cellular surface, tumour cells (e.g., melanoma) secrete high levels of PD-L1 on exosome, a particular form of extracellular vesicle derived from the cellular endocytic pathway, to interfere in systemic immune state.
The research team has developed an amphiphilic metal-phenolic hyaluronic acid nanounit: HACA-Fe@GW4869 nanoparticles (HGF NPs), which can target melanoma cells with CD44 overexpression, decrease the secretion of tumour-derived exosome and weaken the firepower of exosomal PD-L1 indirectly. Immune-active T cells are thus well shielded to secret reactive IFN-γ cytokine, reducing cancer cellular SLC7A11 and SLC3A2, cystine/glutamate transporters. They both show a pivotal role in maintaining the cellular uptake to cystine and glutamate for anti-oxidation per se, whose downregulation inevitably enhances lipid peroxidation. Moreover, enriched cellular iron contributed by HGF NPs further strengthens the cancer cellular ferroptosis. Individual HGF NPs inhibits tumour growth and stimulates long-lasting immunological memory in multiple B16F10 melanoma models. When used in combination with PD-L1 checkpoint blockade, HGF NPs can remedy the therapeutic limitations of free antibodies, including functional optimisation of T cells and suppression of tumour metastasis. The HGF NPs designed is heralded as an attractive candidate for next-generation cancer immunotherapy.
The corresponding author of the related paper is Dr Dai; his PhD student Wang Guohao and postdoctoral fellows Xie Lisi and Li Bei are the co-first authors. Postdoctoral fellow Li Jie and six PhD students—Sang Wei, Yan Jie, Tian Hao, Li Wenxi, Zhang Zhan and Tian Ye—also made important contributions to the study. This project is funded by the Science and Technology Development Fund, Macao SAR (file number: 0109/2018/A3 and 0011/2019/AKP), the UM research fund (file number: SRG2018-00130-FHS), and the Shenzhen-Hong Kong-Macau Science and Technology Programme (file number: SGDX20201103093600004). The full text of the research paper can be viewed at: https://www.nature.com/articles/s41467-021-25990-w