The research teams led by Chen MING, assistant professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), Yingjun ZHAO, professor at Xiamen University, and Wei ZHANG, associate professor in the Department of Neurology at Tangdu Hospital of Air Force Medical University, have made a breakthrough in Alzheimer’s disease (AD) research. They have developed a potentially effective treatment for AD with fewer side effects. The research findings have been published in the top neuroscience journal Neuron.
AD is the most common form of dementia, accounting for around 60-70% of dementia cases, and is one of the leading causes of death in the elderly population. Its pathological features mainly include plaque accumulation, neurofibrillary tangles (NFTs), and brain atrophy caused by neuronal loss. The drugs currently approved for clinical treatment of AD can only moderately alleviate cognitive impairment in AD patients but cannot prevent brain atrophy and neuronal loss. Previous studies have shown that certain forms of phosphorylated tau (p-tau) (such as p-tau181 and p-tau217) increase significantly in the early stages of AD and can be used as biomarkers for early diagnosis of AD, but their role in the onset and progression of AD is still unclear.
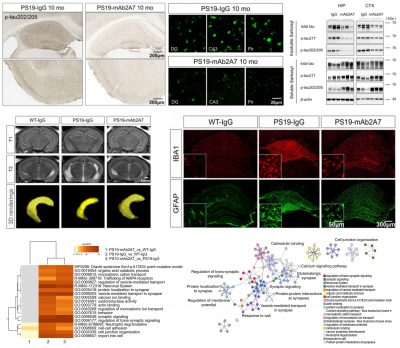
The researchers first screened out the antibody mAb2A7, which specifically targets p-tau217 with high affinity, and found that the antibody could detect neurofibrillary tangles, neuropil threads, and dystrophic neurite-like pathology surrounding Aβ plaques in AD patients’ brain tissue sections. Using single-molecule immunoassay technology (SMID), the researchers found that p-tau217 was elevated in the cerebrospinal fluid and plasma of AD patients compared with non-AD controls. Further analysis revealed that p-tau217 levels in the cerebrospinal fluid of AD patients were positively correlated with their hippocampal atrophy and cognitive impairment, and significantly negatively correlated with the Aβ42/Aβ40 ratio. These results indicate that p-tau217 is closely associated with neurodegenerative changes and amyloid pathology in AD, suggesting that it may be a target for intervention of AD neurodegeneration.
The researchers investigated different methods of antibody administration and found that intranasal administration could deliver the antibodies to the mouse brain and make them bind to the target protein more efficiently. A series of animal experiments and biochemical experiments demonstrated that p-tau217 is a potential target for intervention of AD neurodegenerative changes, and that passive immunotherapy targeting p-tau217 is a potentially effective therapeutic strategy for interfering with AD neurodegeneration with fewer side effects, offering a new approach for the prevention and treatment of AD. Quantitative proteomic analysis further showed that mAb2A7 treatment restored protein homeostasis in the brains of PS19 mice, mainly by upregulating the protein expression of pathways related to synaptic and neuronal function and downregulating the protein expression of oxidative stress and apoptosis-related pathways. These findings underline the importance of restoring protein homeostasis in treating AD.
The study highlights the potential of targeting phosphorylated tau protein for AD treatment, and the fact that targeting p-tau217 has fewer side effects than targeting total tau. The study demonstrates that p-tau217 protein plays a key role in tau pathogenesis and neurodegeneration, and develops a potentially effective immunotherapeutic strategy for AD with fewer side effects. The research findings have attracted much attention in the field of biology. They have been featured in newspapers and journals such as Science and Technology Daily, Xiamen Daily, and Genetics. They have also been widely discussed on many well-known social platforms in the field, such as Alzforum, BioArt, and Biodiscover.
Chen MING, Wei ZHANG, Denghong ZHANG, postdoctoral fellow at Xiamen University, and Xuheng GAO, master’s student at Xiamen University, are co-first authors, and Yingjun ZHAO is the corresponding author. Yunwu ZHANG and Tianying ZHANG, professors at Xiamen University; Chao WANG, professor at Chongqing Medical University; Zhanxiang WANG, professor at the First Affiliated Hospital of Xiamen University; and Huilong YUAN, Xinru MAO, Chunping WANG,Lin SHAN, Zhihao LIN, doctoral students at the First Affiliated Hospital of Xiamen University, also made important contributions to the research. The research teams would like to thank Huaxi XU, professor and founder of the Institute of Neuroscience at Xiamen University, for his advice and guidance during the early stages of the research, as well as the Brain Bank of Chinese Academy of Medical Sciences & Peking Union Medical College and the China Brain Bank of Zhejiang University School of Medicine for providing important patient samples for the study. The research project was supported by UM (File no: SRG2023-00003-FHS). The full version of the research article is available at https://www.cell.com/neuron/fulltext/S0896-6273(24)00127-2.