A research team led by Hanming SHEN, Professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has discovered that spautin-1, an autophagy inhibitor, can target TOMM70, a component of the translocase of the outer mitochondrial membrane (TOMM) complex upon mitochondrial damage, effectively clearing the damaged mitochondria by promoting mitophagy. The study also shows that spautin-1 can significantly improve associative learning ability in an Alzheimer’s disease (AD) Caenorhabditis elegans (C. elegans) model by promoting mitophagy. The research results have been published in the internationally renowned journal Autophagy.
Mitophagy is a form of selective autophagy which removes damaged mitochondria via the autophagy-lysosome pathway. Previous studies have shown that spautin-1 is a well-known autophagy inhibitor that suppresses the function of ubiquitin specific peptidase 10 (USP10) and ubiquitin specific peptidase 13 (USP13) and promotes the degradation of the VPS34-VPS15-BECN1 complex to inhibit bulk autophagy induced by rapamycin or starvation. However, the role of spautin-1 in selective autophagy, such as mitophagy, has not been explored.
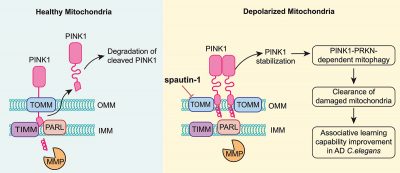
The research team first found that spautin-1 effectively promoted mitophagy to remove damaged mitochondria when mitochondrial damage was induced by various mitophagy inducers (including CCCP, valinomycin, oligomycin/antimycin A). The concentration of spautin-1 used in the experiments did not affect cell survival and mitochondrial glycolysis. Moreover, this process is independent of USP10 and USP13, the known targets of spautin-1. To better understand the mechanism by which spautin-1 promotes mitophagy, the research team focused on PINK1, a key kinase involved in mitophagy, and found that spautin-1 inhibited PINK1 from entering the inner mitochondrial membrane, where it was cleaved and hydrolysed. As a result, PINK1 was stabilised and accumulated at the mitochondrial outer membrane. To further investigate the role of spautin-1 in regulating PINK1, the researchers first used the molecular docking technology to predict the binding ability of spautin-1 with inner and outer mitochondrial membrane transport complexes. The results suggested that spautin-1 may bind to TOMM70, TOMM40, and TOMM20 in the outer mitochondrial membrane transporters. The research team then synthesised biotin-spautin-1 (bio-spautin-1) and confirmed that spautin-1 preferentially bind to TOMM70 using the ‘biotin pulldown’ technique. Next, they used CRISPR-Cas9 technology to generate the TOMM70 knockout cells, and found that the knockout of TOMM70 evidently enhanced the stabilisation and activity of PINK1, which was consistent with the effect of spautin-1. In addition, knockdown of TOMM70 significantly attenuated the effect of spautin-1 in stabilising PINK1, which indicates that TOMM70 is an important target of spautin-1 to stabilise PINK1 and promote mitophagy.
Given that impaired mitophagy is closely associated with the pathogenesis of AD, the research team further explored whether spautin-1 is capable of attenuating the pathogenesis of AD. In the nematode mito-Rosella elegans model, they confirmed that spautin-1 could activate PINK-1-PDR-1-mediated mitophagy (similar to the PINK1-PRKN pathway in mammals) in neurons and improve associative learning ability in an animal model of AD. To conclude, this study identified spautin-1 as a specific mitophagy agonist by positively regulating the stability and activity of PINK1 on the outer mitochondrial membrane, and verified that spautin-1 ameliorates the pathological symptoms of AD in the C. elegans model by promoting mitophagy. Therefore, spautin-1 has the potential for further translational studies.
The corresponding author of this study is Hanming SHEN. The first author is Yi Juan, associate professor in the School of Basic Medical at Lanzhou University. The co-first authors are Guang LU, Associate Professor in the Zhongshan School of Medicine at Sun Yat-sen University, and Heling WANG, doctoral student at the University of Oslo. The study also received substantial support from the team of Rui WANG, vice president of Lanzhou University; Hailong ZHANG, Associate Professor at Lanzhou University; Fei FANG, Associate Professor at University of Oslo; Dajing XIA, Professor at Zhejiang University; Yihua WU, Associate Professor at Zhejiang University; and Liming WANG, Professor at Hunan University. The study was funded by the Science and Technology Development Fund of the Macao SAR (File no: FDCT0031/2021/A1, FDCT0078-2020-A2, FDCT0081/2022/AMJ, and FDCT 0004/2021/1110AKP), UM (SRG2020-00002-FHS, CPG2020-00029-FHS, and CPG2021-00004-FHS), the National Natural Science Foundation of China (File no: 31701206), the Natural Science Foundation of Gansu Province (File no: 20JR5RA281), the Program for the Ministry of Education ‘Peptide Drugs’ Innovation Team (File no: IRT_15R27), and the CAMS Innovation Foundation for Medical Sciences (File no: 2019-I2M-5-074, 2021-I2M-1-026, 2021-I2M-3-001, and 2022-I2M-2-002). The full version of the article is available at https://www.tandfonline.com/doi/full/10.1080/15548627.2024.2383145#abstract.