A research team led by Elaine Lai-Han LEUNG, the professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM), has unveiled critical insights into the responding phenotype of effectiveness of immune checkpoint blockade (ICB) therapy for non-small cell lung cancer (NSCLC) patients. The team identified a set of immune subtypes that are enriched in non-responders to ICB therapy. Moreover, they further elucidated the connection of immune cells with their correlated cytokines. Unlike using biopsy, they provide a noninvasive and feasible method for early and timely monitoring of treatment response. It has been published in the international journal Nature Communications.
Immune checkpoint blockade (ICB) therapy is known to produce durable clinical responses in a selected group of non-small cell lung cancer (NSCLC) patients; therefore, early identification of progression is critical for patient selection. To achieve better effectiveness with ICB therapy, parameters indicating favourable response are critically needed. Several predictors of the response to programmed death-1 (PD-1) blockade have been reported, such as the presence of tumour-infiltrating T cells, high programmed death-ligand 1 (PD-L1) expression in biopsies, microsatellite instability (MSI), Kelch-like ECH-associated protein 1 (KEAP1) and Serine/Threonine kinase 11 (STK11) mutations test and the tumour mutational burden (TMB). However, these parameters are hampered by the limited longitudinal observation window, the small number of parameters and the lack of systematic, unbiased bioinformatic pipelines, which has resulted in a paucity of indicators predicting response state to date. Longitudinal investigation is important to trace markers associated with acquired resistance since the anti-PD1-mediated immune response is dynamic during treatment cycles. However, it is unclear whether noninvasive approaches, such as peripheral blood mononuclear cell (PBMC) profiling, can be used to predict responses to ICB therapy by identifying the relevant responding cell types, which could provide insights for understanding the underlying immunological mechanisms of primary and acquired resistance.
The study, which followed twenty-five NSCLC patients over a period of at least 30 months, harnessed innovative high-dimensional CyTOF and multiplex cytokine approaches. These techniques allowed the researchers to gain deep insights into immune populations, their molecular profiles, and functional characteristics. By analysing immune cell responses, the team identified key markers that can predict the efficacy of anti-PD-1 therapy. The study revealed that the presence of specific immune cell subsets, particularly CD8+CD101hiTIM3+ (CCT T) cells, predicted treatment efficacy. Responding patients exhibited lower frequencies of these subsets, which are associated with exhausted cells. The findings of this study have far-reaching implications for the future of NSCLC treatment. By utilizing advanced techniques to analyse immune responses, researchers have identified novel predictive markers for treatment efficacy. These insights could guide precision therapy and contribute to a better understanding of treatment resistance mechanisms.
Elaine, Lai-Han LEUNG is both the co-corresponding author and first author of the study. Prof. Liang LIU from Second Affiliated Hospital of Guangzhou University of Chinese Medicine, and Dr. Yabing CAO from Macau Kiang Wu Hospital are also the co-corresponding authors, and Prof. Run-Ze LI from Second Affiliated Hospital of Guangzhou University of Chinese Medicine and Prof. Xing-Xing FAN from Macau University of Science and Technology share the first authorship. The research has been supported by the Macau Science and Technology Development Fund (Project no: 0063/2022/A2 & 001/2020/ALC), 2020 Young Qihuang scholar funded by the National Administration of Traditional Chinese Medicine, and Janssen therapeutic fund (Project code: ICD#1101175), The 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab) (Project no: 2020B1212030006), the Start-up Research Grant of University of Macau (SRG2022-00020-FHS) and the Faculty of Health Science, University of Macau, as well as the National Natural Science Foundation of China (82204677). Science and Technology Projects in Guangzhou (SL2022A04J00459) and Technology Research Projects of State Key Laboratory of Dampness Syndrome of Chinese Medicine (no. SZ2022KF20).

Elaine, Lai-Han LEUNG
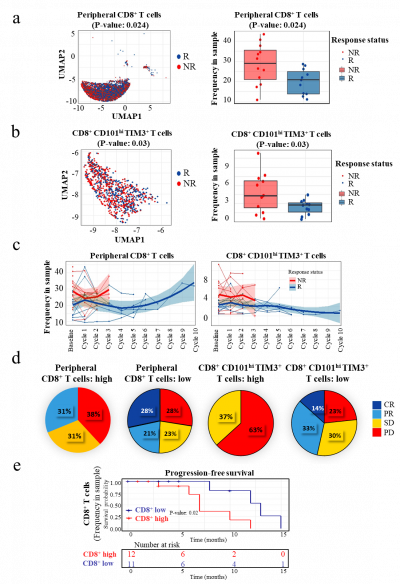
Enrichment of CD8+ and CCT T Subsets: The study revealed that the presence of specific immune cell subsets, particularly CD8+CD101hiTIM3+ (CCT T) cells, predicted treatment efficacy. Responding patients exhibited lower frequencies of these subsets, which are associated with exhausted cells.
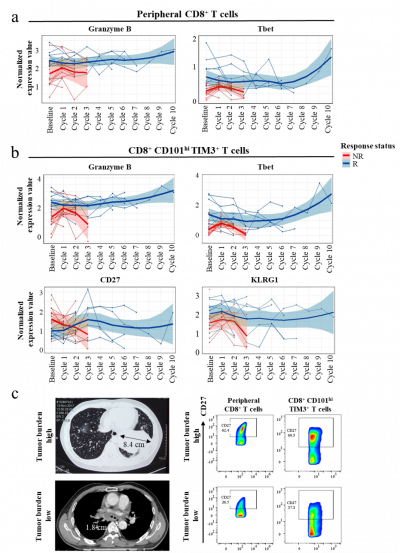
Phenotypic Changes in T Cells: The research uncovered significant changes in the phenotype of CD8+ and CCT T cells among responders. Functional markers like Granzyme B, T-bet, and KLRG1 were found to be upregulated, indicating a potential correlation with positive treatment outcomes.
The findings of this study have far-reaching implications for the future of NSCLC treatment. By utilizing advanced techniques to analyse immune responses, researchers have identified novel predictive markers for treatment efficacy.

