A research team led by Associate Professor Yunlu DAI has designed and synthesized a novel nanomedicine which can achieve robust radiotherapeutic influence and simultaneously relieve the MYC-associated immunosuppression caused by radiation, stimulate systemic anti-tumour immune response and significantly inhibit breast cancer growth, recurrence, and metastasis. The research results have been published in the internationally renowned journal Advanced Materials.
Radiotherapy, although clinically effective in cancer treatment, causes severe immune tolerance and immunosuppression in patients. One main reason is that cancer cells deviously increase the expression of MYC protein after receiving the radiation. This protein possesses the capacity to repair DNA damage caused by radiotherapy, continuing the proliferation of cancer cells and inhibiting the type I interferon (IFN-I) signalling pathway. Impaired IFN-I signalling further impedes antigen presentation, disturbs the activation of cytotoxic T lymphocytes (CTLs), and triggers the expansion of tumour-associated regulatory T (Treg) cells. These cascade effects that MYC overexpression brings in highly inhibit the radiotherapeutic outcomes. Downregulating the MYC level of tumour cells may overcome the critical immunosuppressive barrier and elicit a robust immuno-radiotherapy.
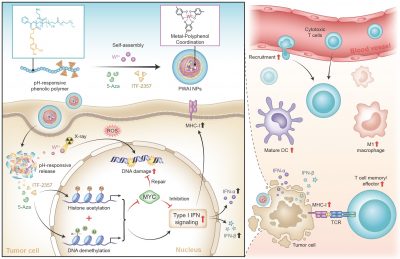
The research team designed an advanced nano-radiosensitizer (PWAI) to launch a dual-epigenetic reprogramming in radiotherapy. The PWAI can coordinate high-Z tungsten metal ions (W6+) with PEG-polyphenols by utilizing a metal-phenolic network and load 5-Aza (DNA methyltransferase inhibitor) and ITF-2357 (histone deacetylase inhibitor) into the hydrophobic core. Under the stimulation of exotic radiation, X ray-sensitive W6+ efficiently destroyed the DNA strands of tumour cells by generating plentiful reactive oxygen species and meanwhile two types of epigenetic inhibitors were released to dual-epigenetically modulate MYC. Intriguingly, although downregulating MYC protein, 5-Aza alone did not obviously trigger IFN-α/IFN-β generation. To combine 5-Aza and ITF-2357, apparent MYC inhibitory effect was still recognized in treated cancer cells, and more importantly, cellular levels of IFN-I signal-related proteins involving IFN-α, IFN-β, and the major histocompatibility complex I were significantly enhanced. To treat 4T1 tumour-bearing mice with PWAI nano-radiosensitizers, the research team identified the awakened anti-tumour immune response after radiotherapy, including the maturation of dendritic cells, cytotoxic T lymphocytes recruitment and their memory-phenotype formation, as well as the immune polarization of tumour-associated macrophages from immunosuppressive state to immunosupportive condition. Bringing the dual-epigenetic reprogramming in radiotherapy may provide a potential strategy for an advanced immuno-radiotherapy.
The corresponding authors of this paper are Yunlu DAI and Bei LI; PhD graduate Guohao WANG, Jie YAN and Hao TIAN are the co-first authors. Postdoctoral fellows Wenxi LI, PhD students Xinying YU, Yuzhao FENG and Songtao ZHOU also made important contributions to the study. This project is funded by the Science and Technology Development Fund, Macao SAR (file number: 0103/2021/A, 0002/2021/AKP, 0133/2022/A3, 0009/2022/AKP and 0006/2023/ITP1), UM (file number: MYRG2022-00011-FHS and MYRG-GRG2023-00013-FHS-UMDF). The full text of the research paper can be viewed at: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.202312588