Eight years ago, the University of Macau (UM) began conducting research in precision oncology. Since then, it has brought together leading scholars from around the world to tackle the major challenges in treating common cancers with high incidence rates, and their work has yielded encouraging results. On 31 December 2020, the university received approval from the Ministry of Education (MoE) to establish a Ministry of Education Frontiers Science Center for Precision Oncology (MoE-FSCPO). As the first national centre of its kind in Hong Kong and Macao, the MoE-FSCPO is committed to leading research and development in life sciences and medicine, especially cancer. A number of preliminary research platforms have been established and collaborations with local and mainland hospitals have been initiated, laying a solid foundation for the development of a leading international platform for precision medicine.
The Only Frontiers Science Center for Precision Oncology in China
By relying on the academic strengths of the ‘double first-class’ universities, the MoE actively establishes world-leading research platforms. Among the ‘double first-class’ universities that have received approval from the MoE to set up frontier science centres in key areas are Tsinghua University and Peking University.
To address the current challenges in cancer treatment, UM established the Faculty of Health Sciences (FHS) in 2013 and put together an interdisciplinary team in the faculty for cancer research and healthcare industry research. The team has been publishing ground-breaking studies, which has earned recognition not only from the academic community but also from the central government.
The MoE-FSCPO is the only frontier science centre in the field of precision oncology approved by the Ministry of Education. The establishment of the centre not only represents a breakthrough of UM in constructing a major national basic research platform, but also shows that the university has reached a new level in the field of precision medicine.
The research team in the MoE-FSCPO is comprised of leading scholars from UM’s Faculty of Health Sciences, Institute of Chinese Medical Sciences, Institute of Applied Physics and Materials Engineering, and Faculty of Science and Technology. They pursue cutting-edge fundamental cancer research and publish breakthroughs in high-impact international journals, attracting attention from the international medical community.
To establish a leading international platform for precision medicine, the MoE-FSCPO has established a number of research platforms in various fields, including cancer tissue and blood banks; multi-omics; big data analysis; high-throughput drug screening, research, and development; drug sensitivity tests; bio-nanomaterials; and animal models. The centre has also worked with local and mainland hospitals to test its research results. According to Prof. Chuxia DENG, a leading Chinese American scientist in the field of life sciences, who is also an internationally renowned expert in cancer research and dean of the FHS, the MoE-FSCPO is China’s only precision oncology centre approved by the Ministry of Education, and it is committed to becoming a leading international centre for innovative research and training of young researchers.
Focusing on Four Areas in Cancer Research
According to the international medical journal The Lancet, in 2020, China recorded 4.57 million new cancer cases, accounting for 23.7 per cent of the total number of cancer cases worldwide. There were also 3 million cancer deaths in China in that year, making it the world’s leading country in terms of new cancer cases and deaths. According to statistics from the Macau Statistics and Census Bureau, Macao had the largest number of deaths from malignant tumours in 2020, accounting for 38.5 per cent of the total number of deaths recorded that year.
For this reason, tackling challenges in treating common cancers with high incidence rates has become a top priority for cancer researchers at UM. According to Prof. Deng, the team has expanded its scope of research to include the prevention, onset, and metastasis of colorectal cancer, lung cancer, and liver cancer, as well as drug resistance among patients of these cancers, in addition to its previous research on breast cancer. The team hopes that by solving key scientific problems, it can substantially improve the accuracy of cancer diagnosis and the effectiveness of cancer treatment.
At present, the MoE-FSCPO is working on four key areas: cancer onset and development, tumour microenvironment and immune regulation, cancer metastasis and drug resistance mechanisms, as well as the development of efficient drugs and personalised cancer treatments. The first area focuses on early diagnosis of tumours to improve the effectiveness of treatment. According to Prof. Deng, Macao has resources to become a large population-based cancer genetics research site given its major population composition of Han with relatively simple genetic background and the long life expectancy of the Macao citizens. The team plans to create a research database based on samples from the local population to improve the accuracy of early cancer diagnosis and prevent cancers caused by genetic mutations.
The second area UM researchers focus on is tumour microenvironment, which is a hot topic in cancer research. The tumour microenvironment is the internal environment in which tumour cells survive and develop, and by interfering with the immune cells in the tumour microenvironment, the team can inhibit cancer development and increase the destruction rate of tumour cells. The third area is a difficult one for cancer researchers, as the vast majority of tumours become drug-resistant after a period of time and most deaths are related to metastasis. The fourth area concerns the efficacy of cancer treatment and support for personalised treatment. ‘Conventional cancer treatment uses a ‘one-size-fits-all’ approach, which requires patients to undergo multiple rounds of physically damaging drug treatments and radiotherapy. We have made it our primary goal to realise personalised treatments, and by treating each individual on a case-by-case basis, we will be able to not only avoid wasting resources but also minimise the cost in terms of lost lives,’ says Prof. Deng.
Precision Medicine for Breast Cancer
Breast cancer is the most commonly diagnosed cancer worldwide. Recurrence and metastasis are the main causes of breast cancer death, and there has been no effective personalised treatment strategy. In a study on breast cancer, a research team led by Prof. Deng and researcher Chen Ping made a breakthrough through collaboration with Kiang Wu Hospital, Conde S Januário Hospital, the Affiliated Hospital of Southwest Medical University, the First Affiliated Hospital of Sun Yat-sen University, Sun Yat-sen University Cancer Center, and Zhuhai People’s Hospital. The research team used patient-derived organoids (PDOs), in vitro drug screening, and other techniques to provide individualised treatment suggestions for breast cancer patients, especially those with advanced breast cancer.
UM researchers generated 99 organoid lines in vitro using tumour samples from breast cancer patients, and developed PDOs as a real-time platform for drug testing. The results showed that breast cancer PDOs could preserve the biological characteristics of primary tumours. PDO pharmaco-phenotyping showed striking variability in the PDO response to the drug treatment. The PDO chemosensitivity profile largely paralleled the retrospective clinical data of the corresponding patients. Finally, PDOs were applied to guide personalised therapy for six patients with advanced cancer, all of whom showed good responses.
Based on the above findings, the team proposed a novel personalised treatment strategy for breast cancer based on drug screening of PDOs, which is a new direction for cancer precision medicine with important clinical significance. The study has been published in Advanced Science, a top international journal with an impact factor of 16.8.
Removing Cancer Cells by Activating the Immune System
Precision oncology is a key area in UM’s research blueprint, which combines the university’s forward-looking research strategies with its cross-disciplinary strengths. The MoE-FSCPO has an interdisciplinary team comprised of leading experts in such areas as health sciences, traditional Chinese medicine, physics and materials, and technological engineering, to conduct basic research on common cancers, with cutting-edge technologies. One of the new directions in cancer treatment pursued by the interdisciplinary team is carbon nanodot cancer immunodiagnosis and treatment. This approach uses a carbon nanodot-based biotechnology to quickly and accurately deliver tumour-specific antigens to the body’s immune lymphatic system, which will then activate the patient’s own immune system to respond to the tumour by removing the cancer cells, thereby achieving a therapeutic effect on the tumour.
Prof. Zikang TANG, director of the Institute of Applied Physics and Materials Engineering and an internationally renowned leader in the field of nanoplasmonic materials, says that cancer is difficult to treat because cancer cells can ‘cunningly escape’ the immune system through an immune escape mechanism. ‘We have collaborated with Prof. Deng’s research team to study the use of a non-toxic, biocompatible carbon quantum dot to ‘facelift’ the proteins of cancer cells so that these cancer proteins no longer resemble normal proteins and can be recognised, engulfed, and antigenically presented by immune cells, in order to achieve a highly efficient immune response,’ he says.
The MoE-FSCPO has conducted extensive animal studies, and the results have demonstrated the power of this quantum biologic tumour immunology technology, which has achieved amazing therapeutic results. A patent application has been filed for the research results and this technology is being tested in clinical trials. ‘This interdisciplinary research project not only has enormous potential for clinical and commercial application, but also brings new hope to cancer patients,’ says Prof. Deng.
Developing a New Biomaterial to Address the Drawbacks of Radiotherapy
Traditional radiotherapy is recognised for its efficacy in treating cancer, but has certain drawbacks, such as the toxicity of high doses of X-rays to normal tissues and the inhibition of toxic substances in the tumour microenvironment during radiotherapy, which limits the effectiveness of radiotherapy. To address this challenge, a team led by Yunlu DAI, assistant professor of FHS and the MoE-FSCPO, has developed a new biomaterial, Hb@Hf-Ce6 nanoparticles, which can directly transport oxygen into deep tumour tissues, reversing the otherwise oxygen-deficient tumour microenvironment and improving the sensitivity of tumour cells to radiotherapy even at low doses. The metal-polyphenol coordination structure of this biomaterial introduces the heavy metal ions into the radiotherapy process and enhances the effectiveness of radiotherapy by generating long-wavelength light and reducing the side effects of toxicity on the human body. ‘Our studies have shown that this material enhances the effectiveness of radiotherapy and radio dynamic therapy, and kills cancer cells in a two-pronged way,’ says Prof. Dai.
In addition, Prof. Dai’s team has developed a gel material based on coordination nanoparticles, which can be applied to tumours in situ after surgery to ensure continuous release of drugs through the material to prevent cancer metastasis. ‘The introduction of PD-1 antibody protein into the structure of this material effectively inhibits distant metastasis of tumours cells into the lung,’ says Prof. Dai. Experiments on mice with ductal carcinoma in situ have shown that this new biomaterial is highly effective in inhibiting tumour growth and metastasis in the long term. The study has been published in Advanced Science.
New Anti-metastatic Therapies
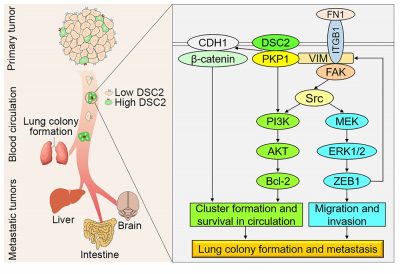
The last thing a patient wants to hear in the fight against cancer is that it has metastasised. When circulating tumour cells (CTCs) try to spread from its original location into the blood vessels, most of them ‘die’ as if they have jumped into a fast stream, unable to withstand the shear stress of the blood flow. If the surviving CTCs succeed in forming metastatic tumours, they will cause death in 90 per cent of the patients. Therefore, it is important to investigate how CTCs survive and metastasise in the bloodstream in order to develop new anti-metastatic therapies. Through the study of molecular mechanisms, a team led by Qian LUO, professor of the FHS and the MoE-FSCPO, has identified a series of molecular targets, such as PKP1 and DSC2, that help CTCs form cell clusters in the circulatory system, thereby increasing their survival rate under the impact of blood circulation and enhancing their ability to metastasise.
‘CTCs survive by bonding together in the blood vessels. PKP1 and DSC2 are cell membrane bridging proteins, and when there are more of these bridging proteins, the CTCs can bond together,’ says Prof. Luo. According to her, the survival rate of CTCs is significantly increased when more of these powerful proteins are present, not just next to each other, but actually ‘hugging each other’ like a raft crossing a river. This finding provides new insights into how CTCs survive in the circulatory system. High expression of PKP1 and DSC2 has been positively correlated with tumour malignancy, and both can be detected in tumour samples from breast and lung cancer patients. This suggests that DSC2 and PKP1 could serve as new biomarkers and therapeutic targets for the detection of metastatic CTCs. The study has been published in the prestigious international journal Science Advances (impact factor 14.14).
Precise Removal of Satellite Tumours
Tumours are often surrounded by small, unidentifiable satellite tumours that are difficult to identify and remove during surgery, while conventional imaging systems such as computed tomography (CT) or magnetic resonance imaging (MRI) are unable to detect these tiny tumours. In view of this, a team led by Tzu-Ming LIU, professor of the FHS and the MoE-FSCPO, has developed an innovative imaging method, the oxygen-sensitive phosphor probe technique, which uses oxygen imaging to identify whether there are any satellite tumours in the vicinity of a primary tumour. Prof. Liu says: ‘Satellite tumours that remain in the body always pose a serious health risk. This fluorescent probe can be inserted into the tissue and emit a light signal where the tissue is deprived of oxygen, indicating the location of the tiny tumour. This enables doctors to accurately remove tumours that are not detected by medical imaging.’
The fluorescent molecular probe is easy to use and does not need to be pre-capped or modified with dendritic molecules. It can be placed directly into the bloodstream by intravenous injection. When the probe penetrates the blood vessel and binds to the collagen fibres surrounding the vessel, the tissue itself becomes an optical readout oxygen concentration metre. The team has conducted a number of in vivo trials, which have shown that the phosphorescent probe can continuously monitor oxygen concentration changes for over six hours, has good stability, and can accurately detect tumours with hypoxic characteristics. The study has been published in Advanced Science.
Souce: My UM e-version
UM establishes internationally advanced precision medicine research platform

UM has established a Frontiers Science Center for Precision Oncology with the approval of the Ministry of Education

A team led by Prof. Chuxia DENG (1st from right) aims to develop new therapies for common cancers with high incidence rates

UM researchers continue to produce groundbreaking research results

Prof. Zikang TANG

The cancer immunotherapy based on carbon-quantum-dots have enormous potential for clinical application and commercialisation.

Prof. Yunlu DAI

UM’s latest study can help develop new anti-metastatic therapies

Prof. Qian LUO
UM’s latest study can help develop new anti-metastatic therapies

Prof. Tzu-Ming LIU

The oxygen-sensitive phosphor probe can accurately detect tumours with hypoxic properties

