A research team led by Prof. Kathy Qian LUO in the Faculty of Health Sciences (FHS) at the University of Macau (UM) has made significant advances in the study of tumour metastasis. The study found that the interaction between macrophages and triple-negative breast cancer (TNBC) cells induces reactive oxygen species (ROS)-mediated interleukin 1α (IL1α) upregulation, which in turn facilitates tumorigenesis and cancer metastasis. This discovery suggests that reducing ROS elevation or downregulating IL1α expression can serve as a new strategy to decrease metastasis of TNBC. The research results have been published in the internationally renowned journal Advanced Science.
TNBC has higher mortality than non-TNBC because of its stronger metastatic capacity. A growing number of studies reported that TNBC tumours had more macrophage infiltration than non-TNBC tumours, which promoted the metastasis of TNBC cells. However, how TNBC cells become more malignant after interacting with macrophages is less reported. Therefore, understanding how TNBC cells and macrophages interact to enhance cancer metastasis and identifying effective therapeutic targets are of great significance for the treatment of TNBC.
In the study, the researchers observed that when TNBC cells were co-cultured with macrophages, they displayed higher viability and stronger metastatic ability than non-TNBC cells. The results of RNA sequencing analysis between monocultured and co-cultured TNBC cells showed that the cytokines IL1α, IL1β and IL8 were highly expressed in co-cultured TNBC cells. High levels of IL1α, IL1β and IL8 are necessary for TNBC cells to better survive when encountering macrophages, achieve stronger metastatic ability, and recruit more tumour-helping M2 macrophages to tumour sites.
Through further mechanistic studies, the researchers discovered that TNBC cells acquire these abilities via interactions with macrophages in three phases. First, within 12 hours of co-culture with macrophages, some TNBC cells significantly elevate levels of ROS, which upregulate IL1α expression in ERK1/2-c-Jun- and NF-κB-dependent manners at 24 to 48 hours. Second, the secreted IL1α bound to IL1R1 activates the ERK1/2-ZEB1-VIM pathway which increases metastasis. Third, IL1α/IL1R1 facilitates its own synthesis and induces the expression of IL1β and IL8 at 72 to 96 hours through the MKK4-JNK-c-Jun and NF-κB signalling pathways. Moreover, a higher level of IL1α is positively correlated with more macrophage infiltration and shorter overall survival in breast cancer patients. Thus, the study suggests that ROS and IL1α may serve as new targets for anti-metastasis therapy in TNBC.
Kathy Qian LUO is the corresponding author of the study and her PhD graduate Hao Meng is the first author. PhD graduates Huang Bin, Wu Renfei, and Peng Zheng also contributed to the study. The research project was supported by the Science and Technology Development Fund of the Macao SAR (File no: 068/2017/A2, 0147/2020/A3, and 0004/2021/AKP), and the Ministry of Education Frontiers Science Center for Precision Oncology of UM (SP2021-00001-FSCPO and SP2023-00001-FSCPO). The full version of the research article can be viewed at: http://doi.org/10.1002/advs.202302857.
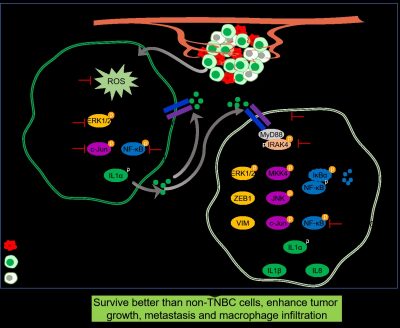
By interacting with macrophages, TNBC cells acquire stronger abilities in cell survival, tumour growth and cancer metastasis

Kathy Qian LUO (1st row, 3rd from left), Meng HAO (1st row, 4th from left), Bin HUANG (2nd row, 2nd from right), Renfei WU (1st row, 1st from right), Zheng PENG (1st row, 1st from left)

