A research team led by Prof. Kathy Qian LUO in the University of Macau (UM) Faculty of Health Sciences (FHS) has found that blood shear stress (SS) triggers mesotrypsin/PRSS3 to cleave and activate protease-activated receptor 2 (PAR2) to facilitate the invasion and metastasis of circulating lung cancer cells. This discovery suggests that PAR2 may be a novel SS-specific mechanosensor in metastasis-initiating circulating tumor cells (CTCs) and provides new insights for developing new anti-metastasis therapies. The study has been published in the internationally renowned journal Advanced Science.
When circulating tumor cells (CTCs) travel in circulation, they can be killed by detachment-induced anoikis and fluidic shear stress (SS)-mediated apoptosis. Circulatory treatment, which can make CTCs detached but also generate SS, can increase metastasis of cancer cells. It has been reported that the suspension state was also able to enhance the cell migration, invasion, and lung metastatic abilities of cancer cells. Therefore, it is necessary to exclude the impacts of detachment to explore the distinct effects of SS on cancer cells.
To identify SS-specific mechanosensors without detachment impacts, a microfluidic circulatory system was used to generate arteriosus SS and compare transcriptome profiles of circulating lung cancer cells with suspended cells. The researchers found that half of the cancer cells could survive SS damage and showed higher invasion ability after circulatory treatment compared with the suspension state. RNA-sequencing analyses showed that mesotrypsin (PRSS3), protease-activated receptor 2 (PAR2), and the subunit of activating protein 1, Fos-related antigen 1 (FOSL1), were upregulated by SS. Further research confirmed that their high expression was responsible for promoting invasion and metastasis of lung cancer cells.
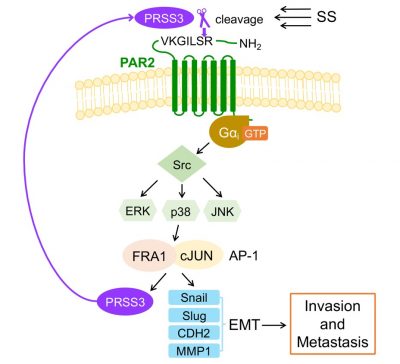
The researchers then found that SS could trigger PRSS3 to cleave the N-terminal inhibitory domain of PAR2 within 2 h. As a G protein-coupled receptor, PAR2 further activated the Gαi protein to turn on the Src-ERK/p38/JNK-FRA1/cJUN axis to promote the expression of epithelial–mesenchymal transition markers, and also PRSS3, which facilitated metastasis. Enriched PRSS3, PAR2, and FOSL1 in human tumor samples and their correlations with worse outcomes reveal their clinical significance. Therefore, PAR2 may serve as an SS-specific mechanosensor cleavable by PRSS3 in circulation, which provides new insights for targeting metastasis-initiating CTCs.
The corresponding author of this study is Kathy Qian LUO. Her PhD student Muya ZHOU is the first author and Koukou LI is the second author. This work was supported by the Multi-Year Research Grant (MYRG2018-00092-FHS), the Ministry of Education Frontiers Science Center for Precision Oncology (SP2021-00001-FSCPO and SP2023-00001-FSCPO) from the University of Macau, and the Science and Technology Development Fund of Macao (FDCT [0147/2020/A3] and FDCT Key Project [0004/2021/AKP]). The full text of the research article can be viewed at: https://doi.org/10.1002/advs.202301059.