A research team led by Henry Hang Fai KWOK, Associate Professor at the University of Macau (UM) Faculty of Health Sciences (FHS), has made significant new progress in a novel application strategy for the anti-cancer drug Homoharringtonine (HHT). The research team has clarified the mechanisms underlying HHT activity against bladder cancer growth, which indicates that HHT shows enormous potential as an anti-cancer agent and could be applied as a combination treatment strategy for bladder cancer. The research results have been published in the internationally renowned journal Pharmacological Research.
Bladder cancer is the tenth most commonly diagnosed cancer worldwide, 25% of the patients were diagnosed with invasive bladder cancer or metastasis disease for the first time, and the 5-year survival rate was only 5%. Once the patients are diagnosed with bladder cancer, they will face limited treatment options and a complex, long-term care pathway.Furthermore, bladder cancer has the highest recurrence rate amount all cancer types. The patients’ living quality is worse and the therapy is more complicated.
Applying immune checkpoint inhibitors and FGFR protein tyrosine kinase inhibitors in bladder cancer therapy provides an opportunity to improve outcomes for patients with high-grade metastatic cancer. However, most bladder cancer patients in all stages do not respond to immunotherapy, and drug-resistant problems occur highly in patients with recurrence. There is an urgent need for renewal agents or treatments as new options for patients with bladder cancer. For a new drug to enter the market, there is a long development cycle with high costs and low success rates. Repurposing Food and Drug Administration (FDA) previously approved medications and using them for novel drug combination strategies are probably one of the best and fastest ways to tackle this kind of hard-to-treat cancer type.
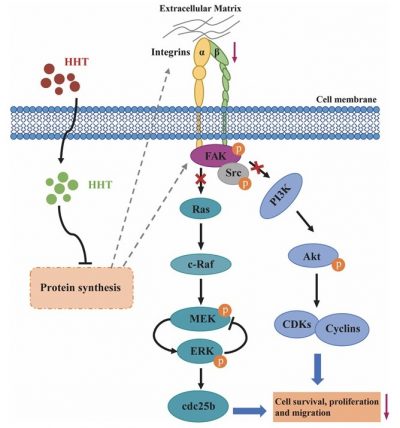
Homoharringtonine (HHT) has been used for hematologic malignancies for over 40 years in China. The FDA approved HHT in 2012 as an approved anti-leukemia drug. Many studies have demonstrated that HHT effectively inhibits the development of several types of solid tumors, although the underlying mechanisms of action are unclear. In this study, Prof. Kwok’s research team investigated the mechanisms underlying HHT activity against bladder cancer growth. The research team compared HTT with the drugs currently used clinically for bladder cancer treatment. HHT showed more potent inhibitory activity than cisplatin, carboplatin, and doxorubicin. The in vitro and in vivo data demonstrated that HHT inhibited proliferation, colony formation, migration, and cell adhesion of bladder cancer cells and induced apoptosis and cell cycle arrest in the nanomolar concentration range. Furthermore, the research team revealed that HHT treatment could downregulate the MAPK/Erk and PI3k/Akt signaling pathways by inactivating the integrin α5/β1-FAK/Src axis. In addition, HHT-induced activity reduced cell-extracellular matrix (ECM) interactions and cell migration, thus suppressing tumor metastasis progression.
Prof. Henry Hang Fai KWOK is the corresponding author of this study, and his PhD student Qiushuang WU is the first author. In addition, Prof. Qingwen ZHANG from UM Institute of Chinese Medical Sciences (ICMS) has also made important contributions to this study. This study was supported by the Science and Technology Development Fund, Macao SAR (file no.: 0010/2021/AFJ, 0027/2022/A1), the Guangzhou Science and Technology Innovation Funding (file no.: 201807010096), UM ( file no.: MYRG2019-00150-ICMS), the Guangdong-Hong Kong-Macao Research Team Project of the Guangdong Basic and Applied Basic Research Foundation (file no.: 2022B1515130008). The full version of the research study can be viewed at https://doi.org/10.1016/j.phrs.2023.106654