A research team led by Hongjie ZHANG, associate professor in the Faculty of Health Sciences (FHS), University of Macau (UM), has made significant progress in understanding how the structures and functions of organs are maintained. Prof. Zhang’s team has discovered that an imbalance of cellular protein homeostasis and autophagy overactivation is the major cause of intestinal damage and dysfunction upon loss of a novel protein RIKE-1. Given that autophagy-mediated protein quality control is involved in the pathogenesis of a broad range of diseases and conditions, such as neurodegeneration, cancer and aging, the discovery holds promise for identifying therapeutic intervention targets for slowing down the progression of these diseases and aging. The study has been published in the renowned international journal Autophagy.
Autophagy is an evolutionarily conserved degradation system, serving to clean out the damaged and/or unwanted components within cells. Autophagy occurs naturally at a basal level in most cells to maintain cellular homeostasis and fitness. Autophagy can be induced by many types of stresses, such as starvation and organelle/DNA damage. The autophagic response needs to be tightly regulated because its level is often linked with beneficial or detrimental outcomes. While a moderate amount of autophagy activation protects organisms from degenerative, inflammatory and infectious diseases, a persistent and overactivated autophagic response promotes cell death and organismal damage. The stimuli that trigger autophagy overactivation and its cytotoxic effect in vivo remain largely unknown. A mechanistic understanding of autophagy overactivation and its impact is critical to precisely control the level of autophagy for therapeutic intervention in many diseases.
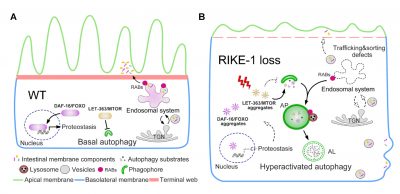
The exploration of the mechanisms underlying autophagy overactivation by Prof. Zhang’s team began with their studies in C. elegans intestinal development. While seeking molecules that affect intestinal development, they found that animals lacking the Kelch repeat-containing protein RIKE-1, encoded by a previously uncharacterized gene, C53A5.6, exhibit severe defects in intestinal morphology and integrity accompanied by mislocalization of intestinal membrane components and excessive endosomal degradation. They identified that autophagy overactivation is the underlying cause of excessive endosomal degradation. Further study reveals that autophagy overactivation is caused by imbalanced proteostasis, which consequently leads to cytoplasmic aggregation of proteins, including LET-363/MTOR. Attenuating the level of DAF-2 insulin/IGF1 signaling alleviates the LET-363/MTOR aggregation and autophagy overactivation, which in turn restores epithelial morphology in RIKE-1-deficient animals. Their findings suggest a role of RIKE-1 in maintaining the basal level of autophagy and epithelial integrity through shaping the cellular proteome.
Prof. Zhang is the corresponding author of the study, and PhD student Cheng Xiaoxiang is the first author. Two other PhD students of the FHS, namely Pei ZHANG and Kai ZHENG, and Prof. Hong ZHANG from the Chinese Academy of Science, as well as his lab members, Hongyu ZHAO and Hui ZHENG, also contributed to this project. The project was supported by the Science and Technology Development Fund, Macao SAR (File no.: 018/2017/AMJ, 050/2018/A2 and 0136/2020/A3) and UM’s research fund (File no.: MYRG2017-00082-FHS, MYRG2019-00063-FHS and MYRG2020-00163-FHS). The full version of the research article can be viewed at https://www.tandfonline.com/doi/full/10.1080/15548627.2022.2071381