A team led by Yunlu DAI, the associate professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM), has made a significant progress in cancer immunotherapy. This study fabricates a new metal-polyphenol nanomedicine to regulate cancer cell metabolism by means of ultrasound-mediated oxidative stress and aerobic glycolysis suppression. This “sono-metabolic” treatment helps reprogramme tumour microenvironment, thereby improving T-cell antitumour efficacy. It has been published in the international journal ACS Nano.
Cellular metabolism is crucial for maintaining the viability and function of cancer cells and immune cells. The classic metabolic pathway in cancer cells is the “aerobic glycolysis” in which high glycolytic rates exhibited in most cancer cells regardless of oxygen availability. This is a hallmark property of malignancy. It has been reported that cancer cells with high metabolic activity outcompete the adjacent immune cells with nutrients. The high glucose utilization and lactate production of cancer cells not only deprives CD8+ T cells of energy source and impair their effector functions, but also supports regulatory T (Treg) cell immunosuppressive activity.
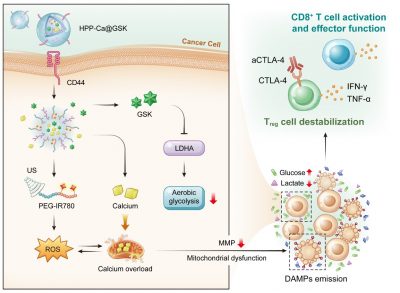
Hence, a novel metal-phenolic nanomedicine, HPP-Ca@GSK, has been developed to perform efficient cancer cellular mitochondrial dysfunction and tumour microenvironment reprogramming via a sono-metabolic treatment. Specifically, functional GSK (LDHA inhibitor) suppresses cancer glycolytic metabolism and recreates a high-glucose, low-lactate tumour microenvironment, which benefits CD8+ tumour-infiltrating T lymphocyte effector function and Treg cell destabilization. To further overload intracellular calcium coupled with ultrasound-mediated oxidative stress, the mitochondrial disorder is triggered for highly efficient damage-associated molecular patterns exposure. This assists dendritic cell maturation and CD8+ T cell activation, enabling more tumour infiltration of effective CD8+ T cells. Last but not least, the assistance of aCTLA-4 antibodies further amplifies the sono-metabolic therapeutic influence.
Yunlu DAI and FHS Research Assistant Professor Bei LI are the corresponding authors of the study, and PhD student Jie YAN, FHS research assistant Wenxi LI, and PhD student Hao TIAN are the co-first authors. PhD student Xinying YU, PhD graduates Guohao WANG and Wei SANG also have made important contributions to the study. The Proteomics, Metabolomics and Drug Development Core, Animal Research Core, and Biological Imaging and Stem Cell Core of FHS have also provided important support for the research. The work was supported by the National Natural Science Foundation of China (file number: 32222090, 32171318 and 32101069), the University of Macau (file number: MYRG2022-00011-FHS), the Science and Technology Development Fund, Macau SAR (file number: 0103/2021/A, 0002/2021/AKP and 0133/2022/A3), Shenzhen-Hong Kong-Macau Science and Technology Plan C (file number: SGDX20201103093600004), Dr. Stanley Ho Medical Development Foundation (file number: SHMDF-OIRFS/2022/002) and Ministry of Education Frontiers Science Centre for Precision Oncology, University of Macau (file number: SP2023-00001-FSCPO). The full version of the research is available at https://pubs.acs.org/doi/full/10.1021/acsnano.3c02428.